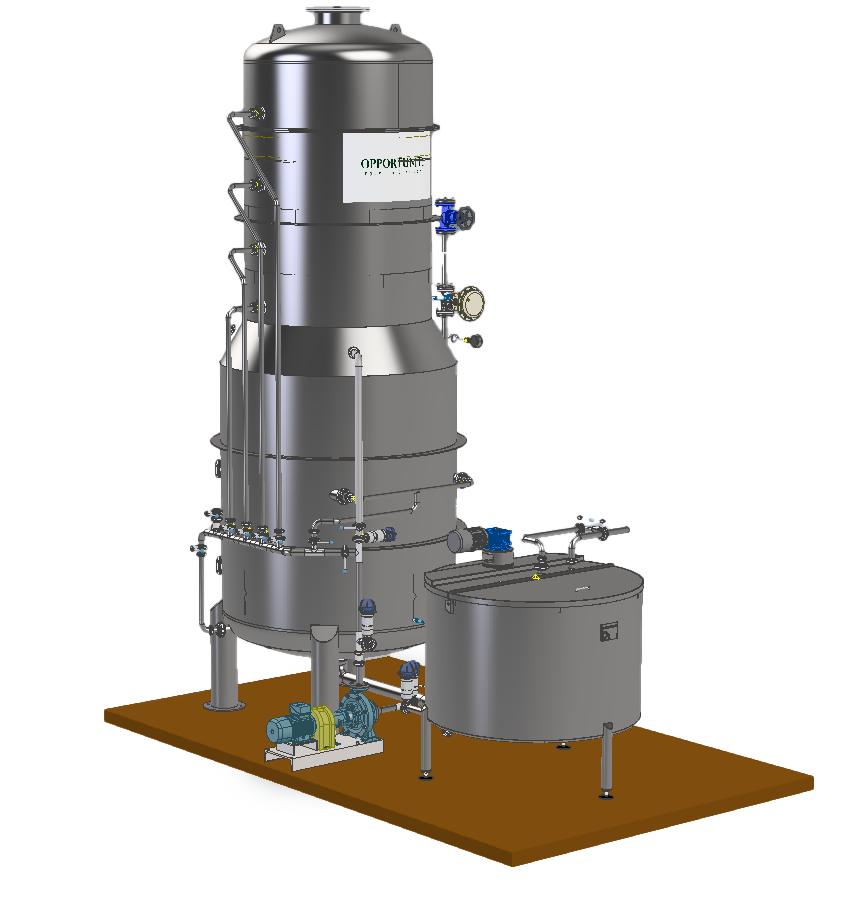
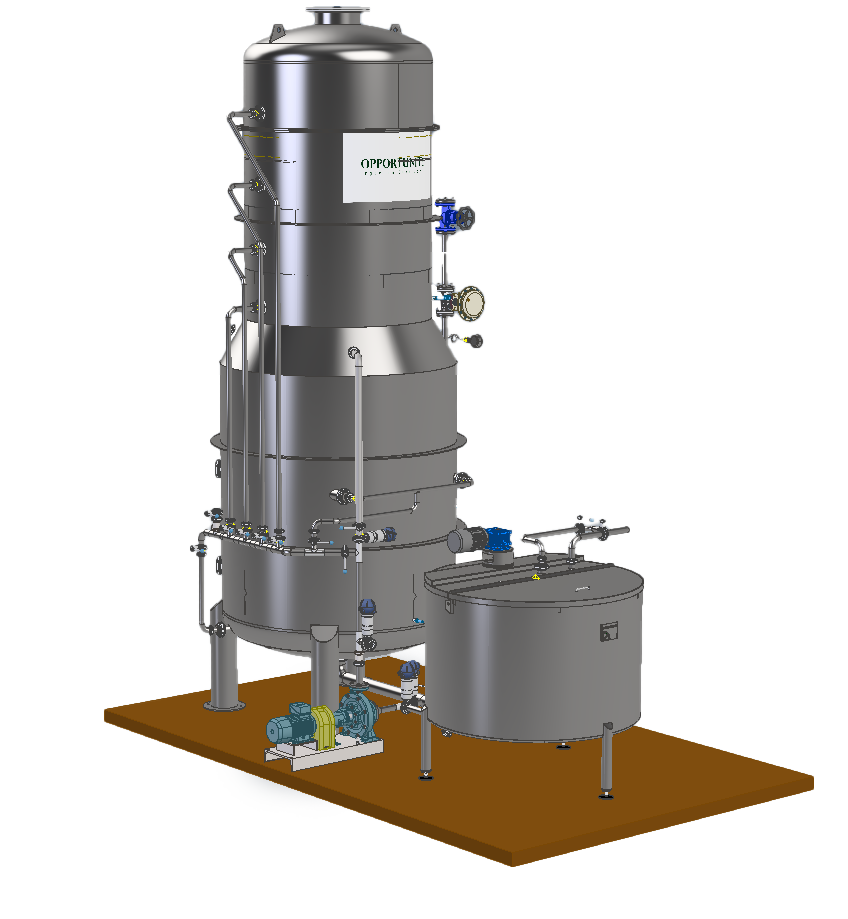
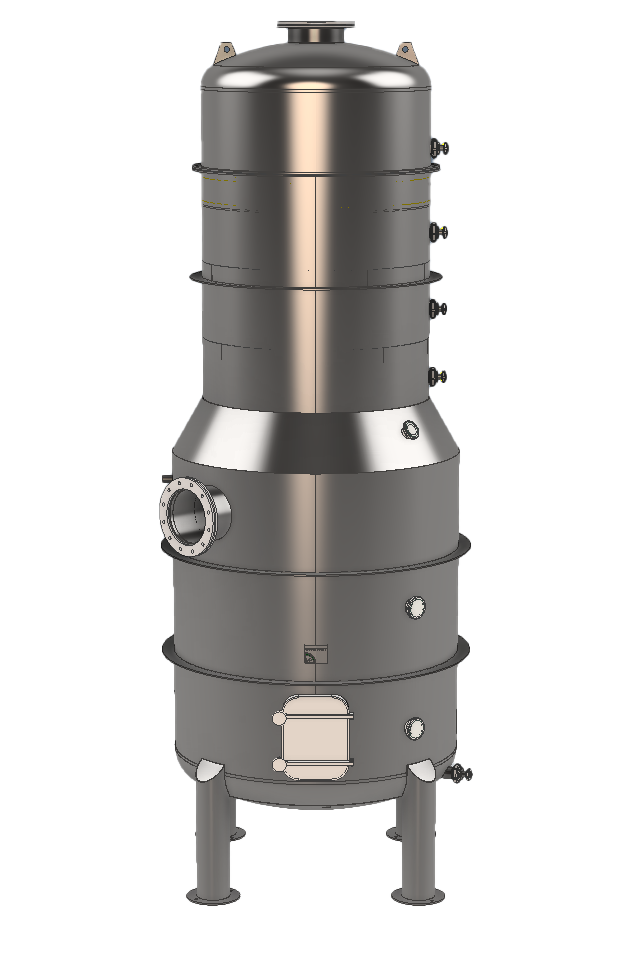
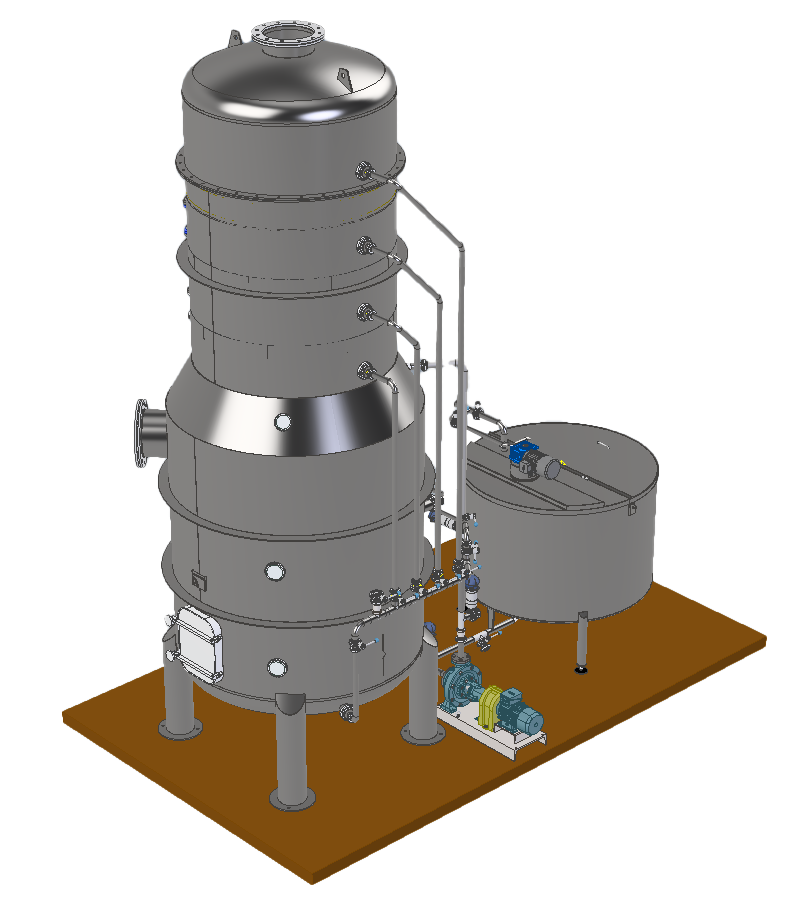
HOW IT WORKS:
The evaporation of sulphur dioxide is achieved through a stream of plant vapour, generated by the must itself, and recirculated through a reliable mechanical recompression system.
This takes place in a plate column where countercurrent contact is made between the must being treated and the ascending recompressed vapour.
The vapour, rich in SO2, is bubbled into a bubbler, contained in a neutraliser, in which an instantaneous chemical reaction breaks down 100% of the SO2 present.
TREATMENT CHARACTERISTICS:
The treatment of the product is extremely rapid, and therefore exposure to the process temperature is minimal.
The recovery of neutralised vapours ensures that the aromas present in the product to be treated are not lost.
In order to achieve optimal sulphur dioxide elimination (less than 10 ppm), the product must be well prepared at the time of processing and subsequently well stored.
Any anamole fermentation in an environment rich in SO2 and thus poor in oxygen generates compounds that prevent the SO2 from evaporating and thus being eliminated.